Nous vous aidons à
évaluer améliorer concevoir sécuriser
vos produits solaires
WENEOS (ex HelioScreen) est le centre expert dans l’évaluation de la protection solaire de produits cosmétiques.
Résistance à la Sueur
UVA Ultra-Long
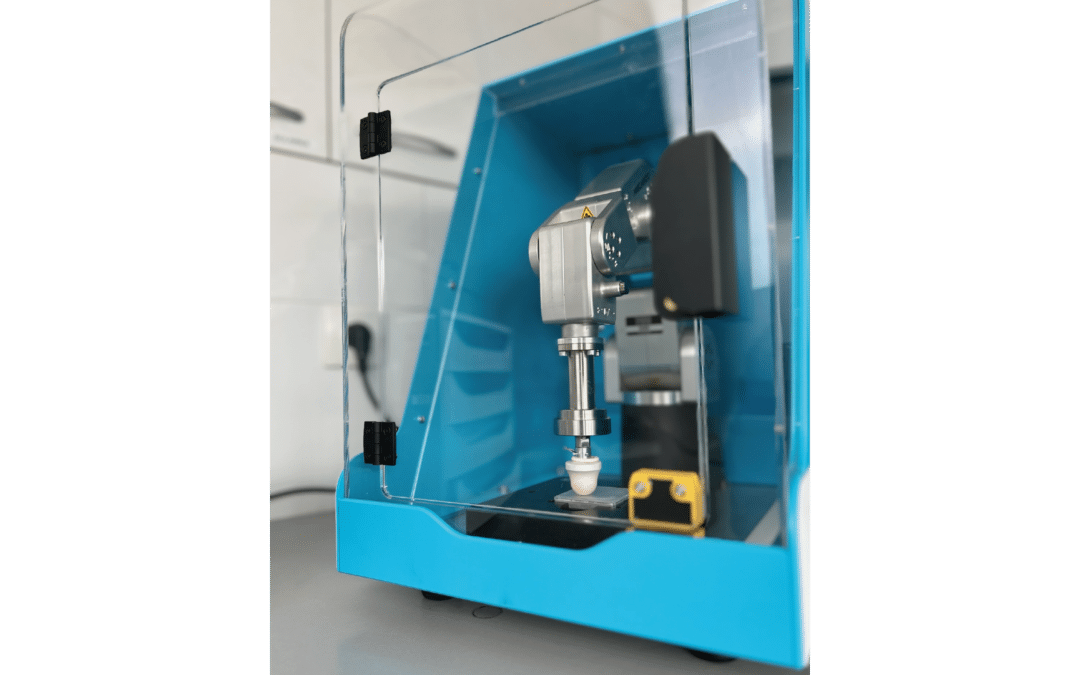
SPREADMASTER
HDRS-Poly602
Déjà + de 500 entreprises nous font confiance
Notre expertise au service de la protection solaire
Évaluation solaire
- SPF | UVA | LOC | HEV | IR
- Sueur | Sable | Eau | Rub | Wet
- Contrôle | Absorbance
Substrat PMMA
- Moulée HD6
- Sablée SB6
Equipement in vitro
- SPREADMASTER
- THERMASTER
- Simulateur solaire | Radiomètre
Equipement in vivo
- HDRS | Poly602 | Mono602
- Simulateur solaire | Radiomètre
Référence
- P2 | P5 | P8 | S2
Formation solaire
- Théorie
- Pratique
- Technique
Exiger un niveau de qualité élevé pour tester vos produits solaires
WENEOS en quelques chiffres
d'expérience
tests réalisées
en surface totale
employé(e)s
Vos résultats in vitro pour un produit en moins de 7 jours
Réception du produit solaire
Réalisation de l'étude solaire
Rapport final
Nos dernières actualités
Distribution des nouveaux appareils HDRS
Les méthodes SPF non invasives de spectroscopie de réflectance diffuse hybride (HDRS), conformément à la norme proposée ISO/CD 23698, sont actuellement évaluées comme alternative aux tests SPF in vivo pour fournir des valeurs…
Nouveau SPREADMASTER, plus précis et plus comptact
Il y a 10 ans, nous avions révolutionné le marché de l'évaluation in vitro de la protection solaire avec l'étalement robotisé via le HD-SPREADMASTER. Sa capacité à assurer un niveau…
Changement d’identité – HelioScreen devient WENEOS
WENEOS, notre nouveau nom pour une mission plus large L'identité d'une entreprise se manifeste à travers son nom, révélateur de ses valeurs et ambitions. Changer de nom représente donc…